An open access journal
An open access journal
Causal Association Between Leukocyte Telomere Length and Psoriasis: A Two-Sample Bidirectional Mendelian Randomization Study
Abstract
Background: Observational studies have demonstrated a relationship between leukocyte telomere length (LTL) and psoriasis. However, the causal association between them remains unknown.
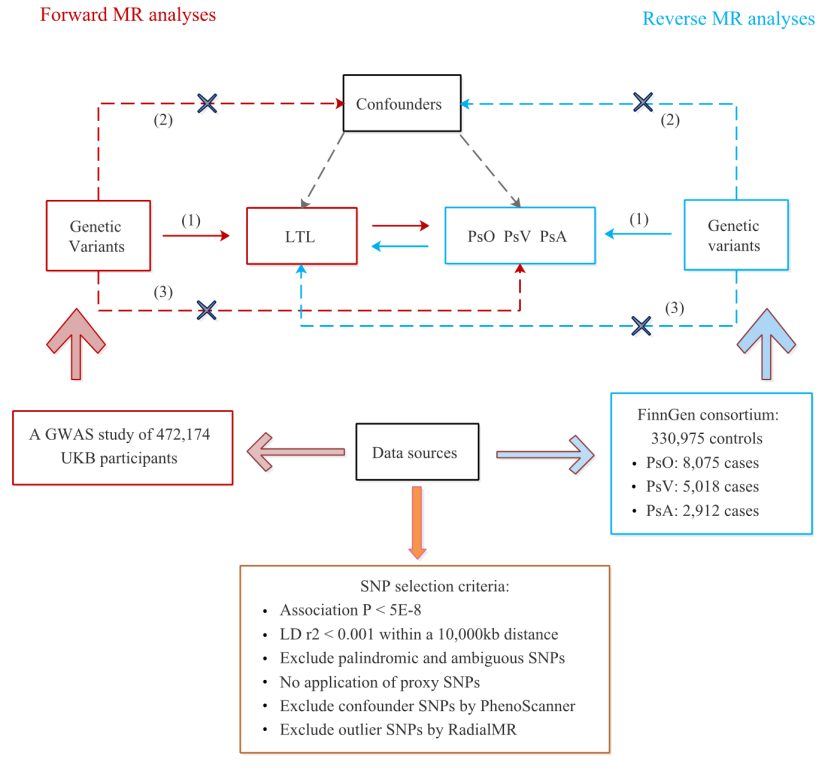
Methods: Bidirectional Mendelian randomization (MR) analysis was performed using publicly available genome-wide association study (GWAS) summary statistics. The GWAS summary data involving 472,174 participants for ITL were obtained from the UKbiobank, while the summary data for psoriasis (8,075 cases) and its two main types, psoriasis vulgaris (PsV, 5,018 cases) and psoriatic arthritis (PsA, 2,919 cases) were obtained from the FinnGen consortium. The inverse variance weighted (IVW) method was used as the main analysis.
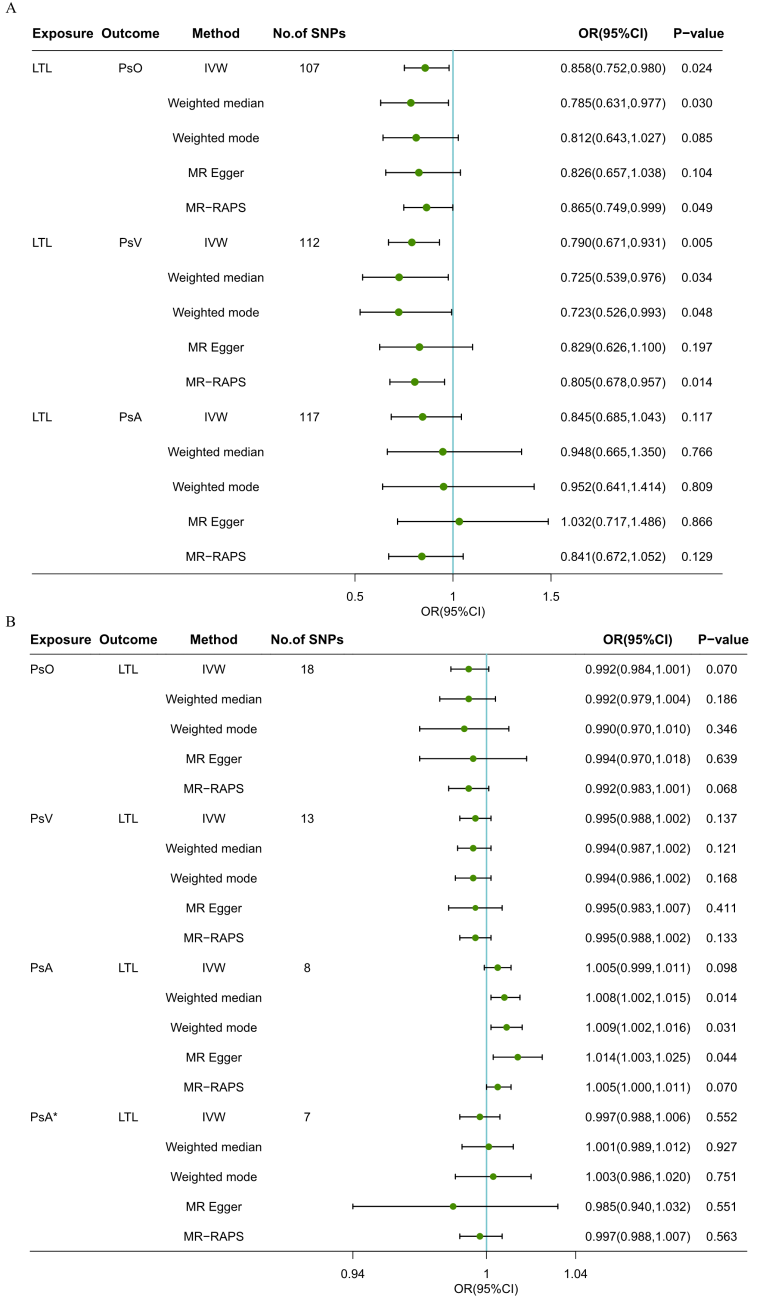
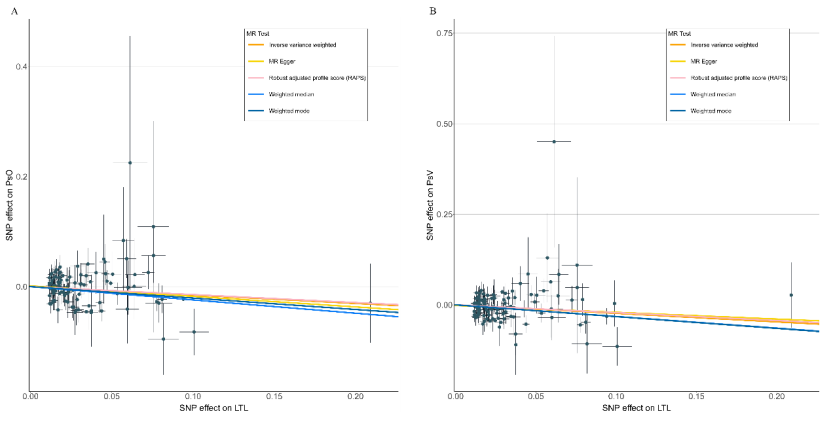
Results: The forward MR analyses indicated that genetically determined LTL was suggestively associated with psoriasis (IVW: OR: 0.858; 95% CI: 0.752-0.980; P= 0.024) and significantly associated with PsV (IVW: OR: 0.790; 95% CI: 0.671-0.931; P= 0.005), but not with PsA (IVW: OR: 0.845; 95% CI: 0.685-1.043; p= 0.117). The reverse MR analyses showed that no causal effects were observed in psoriasis and its two subtypes on LTL.
Conclusion: Our study suggested a potential causal effect of shortened LTL on the increased risk of psoriasis, especially PsV, providing LTL as a novel biomarker and therapeutic target for psoriasis.
Show Figures
Share and Cite
Article Metrics
References
- NPF. Psoriasis statistics available from National Psoriasis Foundation. December 21, 2022; Available from: https://www.psoriasis.org/psoriasis-statistics/.
- Griffiths, C.E.M., et al., Psoriasis. Lancet (London, England), 2021. 397(10281): p. 1301-1315.
- Chekol Abebe, E., et al., Role of Fetuin-A in the Pathogenesis of Psoriasis and Its Potential Clinical Applications. Clinical, Cosmetic and Investigational Dermatology, 2022. 15: p. 595-607.
- Langley, R.G.B., G.G. Krueger, and C.E.M. Griffiths, Psoriasis: epidemiology, clinical features, and quality of life. Annals of the Rheumatic Diseases, 2005. 64 Suppl 2(Suppl 2).
- Alinaghi, F., et al., Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. Journal of the American Academy of Dermatology, 2019. 80(1).
- Eder, L., et al., The Incidence and Risk Factors for Psoriatic Arthritis in Patients With Psoriasis: A Prospective Cohort Study. Arthritis & Rheumatology (Hoboken, N.J.), 2016. 68(4): p. 915-923.
- Takeshita, J., et al., Psoriasis and comorbid diseases: Epidemiology. Journal of the American Academy of Dermatology, 2017. 76(3): p. 377-390.
- Molinuevo, R., et al., The DNA damage response links human squamous proliferation with differentiation. The Journal of Cell Biology, 2020. 219(11).
- Demanelis, K., et al., Determinants of telomere length across human tissues. Science (New York, N.Y.), 2020. 369(6509).
- Blackburn, E.H., Switching and signaling at the telomere. Cell, 2001. 106(6): p. 661-673.
- Zhang, J., et al., Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Research Reviews, 2016. 25: p. 55-69.
- O'Donovan, A., et al., Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PloS One, 2011. 6(5): p. e19687.
- Hohensinner, P.J., J.J. Goronzy, and C.M. Weyand, Telomere dysfunction, autoimmunity and aging. Aging and Disease, 2011. 2(6): p. 524-537.
- Zeng, Z., et al., Association of telomere length with risk of rheumatoid arthritis: a meta-analysis and Mendelian randomization. Rheumatology (Oxford, England), 2020. 59(5): p. 940-947.
- Liao, Q., et al., A causal relationship between leukocyte telomere length and multiple sclerosis: A Mendelian randomization study. Frontiers In Immunology, 2022. 13: p. 922922.
- Wu, K., et al., Telomerase activity is increased and telomere length shortened in T cells from blood of patients with atopic dermatitis and psoriasis. Journal of Immunology (Baltimore, Md. : 1950), 2000. 165(8): p. 4742-4747.
- Tamayo, M., et al., Differing patterns of peripheral blood leukocyte telomere length in rheumatologic diseases. Mutation Research, 2010. 683(1-2): p. 68-73.
- Beranek, M., et al., Telomere length, oxidative and epigenetic changes in blood DNA of patients with exacerbated psoriasis vulgaris. Anais Brasileiros de Dermatologia, 2023. 98(1): p. 68-74.
- Davies, N.M., M.V. Holmes, and G. Davey Smith, Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (Clinical Research ed.), 2018. 362: p. k601.
- Burgess, S., A. Butterworth, and S.G. Thompson, Mendelian randomization analysis with multiple genetic variants using summarized data. Genetic Epidemiology, 2013. 37(7): p. 658-665.
- Codd, V., et al., Polygenic basis and biomedical consequences of telomere length variation. Nature Genetics, 2021. 53(10): p. 1425-1433.
- Kurki MI, K.J., Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: unique genetic insights from combining isolated population and national health register data. medRxiv. 2022;2022.03.03.22271360. Available from: https://doi.org/10.1101/2022.03.03.22271360.
- FinnGen. {FinnGen} Documentation of R8 release. 2022; Available from: https://finngen.gitbook.io/documentation/.
- Kamat, M.A., et al., PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics (Oxford, England), 2019. 35(22): p. 4851-4853.
- Müezzinler, A., A.K. Zaineddin, and H. Brenner, A systematic review of leukocyte telomere length and age in adults. Ageing Research Reviews, 2013. 12(2): p. 509-519.
- Bowden, J., et al., Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. International Journal of Epidemiology, 2018. 47(4): p. 1264-1278.
- Burgess, S. and S.G. Thompson, Avoiding bias from weak instruments in Mendelian randomization studies. International Journal of Epidemiology, 2011. 40(3): p. 755-764.
- Bowden, J., et al., Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genetic Epidemiology, 2016. 40(4): p. 304-314.
- Hartwig, F.P., G. Davey Smith, and J. Bowden, Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. International Journal of Epidemiology, 2017. 46(6): p. 1985-1998.
- Bowden, J., G. Davey Smith, and S. Burgess, Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. International Journal of Epidemiology, 2015. 44(2): p. 512-525.
- Zhao, Q., et al., Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. The Annals of Statistics, 2020. 48(3): p. 1742-1769, 28.
- Bowden, J., et al., A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Statistics In Medicine, 2017. 36(11): p. 1783-1802.
- Verbanck, M., et al., Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics, 2018. 50(5): p. 693-698.
- Brion, M.-J.A., K. Shakhbazov, and P.M. Visscher, Calculating statistical power in Mendelian randomization studies. International Journal of Epidemiology, 2013. 42(5): p. 1497-1501.
- Ramunas, J., et al., Transient delivery of modified mRNA encoding TERT rapidly extends telomeres in human cells. FASEB Journal : Official Publication of the Federation of American Societies For Experimental Biology, 2015. 29(5): p. 1930-1939.
- Townsley, D.M., et al., Danazol Treatment for Telomere Diseases. The New England Journal of Medicine, 2016. 374(20): p. 1922-1931.
- Haycock, P.C., et al., Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncology, 2017. 3(5): p. 636-651.
- Kaszubowska, L., Telomere shortening and ageing of the immune system. Journal of Physiology and Pharmacology : an Official Journal of the Polish Physiological Society, 2008. 59 Suppl 9: p. 169-186.
- Matthe, D.M., et al., Telomerase deficiency reflects age-associated changes in CD4+ T cells. Immunity & Ageing : I & A, 2022. 19(1): p. 16.
- Childs, B.G., et al., Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nature Medicine, 2015. 21(12): p. 1424-1435.
- Effros, R.B., Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Experimental Gerontology, 2011. 46(2-3): p. 135-140.
- Salminen, A., A. Kauppinen, and K. Kaarniranta, Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cellular Signalling, 2012. 24(4): p. 835-845.
- Velarde, M.C., M. Demaria, and J. Campisi, Senescent cells and their secretory phenotype as targets for cancer therapy. Interdisciplinary Topics In Gerontology, 2013. 38: p. 17-27.
- Armstrong, A.W., Psoriasis. JAMA Dermatology, 2017. 153(9): p. 956.